Which of the Following Best Describes a Double Replacement Reaction
B NH4Cl NH3 HCl. Zns CuSO4aq -.

Decomposition Reactions Youtube Chemical Reactions Chemical Equation Reactions
What term refers to a chemical reaction that absorbs heat energy.

. Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube. D 2 Mg O2 2 MgO. This is a double replacement reaction that is also a neutralization It is a double replacement because the reaction starts with two compounds and ends with two compounds where the positive and negative ions have changed places.
2HClaq CaCO3s CO2g CaCl2aq H2Ol. In this type of reaction the positive-charged cations and the negative-charged anions of the reactants both trade places double displacement to form two new products. Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds.
Identify the precipitate formed when solutions of the following ionic compounds are mixed. AB CD AD CB. C the reactants are usually a metal and a nonmetal.
A gas us detected a precipitate is formed a flame is observed. C In a decomposition reaction. A the reactants are generally two ionic compounds in aqueous solution.
B energy in the form of heat or light is often produced. This group of writers have passed strict English tests plus tests from their fields of specialization. What are the products from the following single-replacement reaction.
What term refers to a chemical reaction that absorbs heat energy. Write a sentence that completely describes the chemical reaction represented by this balanced equation. Double-replacement reactions generally occur between substances in aqueous solution.
This means they recently joined the team. Double replacement reaction O acid base reaction. The positive hydrogen ion on the Chlorine has been replaced by a positive sodium ion on the Chlorine.
Thats why we take the recruitment process seriously to have a. See answer 1 Best Answer. AB A B A B AB A BC AC B AC BD AD BC.
A 2 N2 3 H2 2 NH3. Double replacement reactions take the form. A B - C D - A D - C B -.
Which of the following best describes a double-replacement reaction. In this reaction A and C are positively-charged cations while B and D are negatively-charged anions. The double-replacement reaction below results in the formation of the precipitate lead.
The the following reaction is. For a chemical reaction. Which of the following best represents a double replacement reaction.
The general form of a double-replacement also called double-displacement reaction is. Draw curved arrows for the following reaction step. A double displacement reaction is also known as salt metathesis reaction double replacement reaction exchange or sometimes a double decomposition reaction although that term is used when one or more of the reactants does not dissolve in the solvent.
Atoms in one compound switch places with atoms in another compound. Which of the statements below best describes the following reaction. C Cd NO32 Na2S CdS 2 NaNO3.

Combustion Reactions Chemical Reactions Chemical Equation Reactions

A Synthesis Reaction Is A Type Of Reaction In Which Multiple Reactants Combine To Form A Single Product Synthesis React Chemical Reactions Chemistry Synthesis

Exothermic Reactions Release Energy Endothermic Reactions Consume Energy Exothermic Reaction Homeschool Science Chemistry
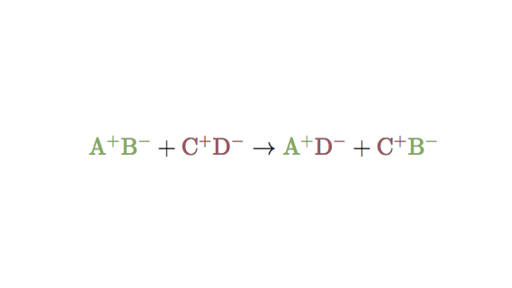
Double Replacement Reactions Double Displacement Article Khan Academy
Comments
Post a Comment